Introducing the Danube II
Fluorescence Lifetime Flow Cytometer
In cell biology and cancer research, there is often the need to measure cellular processes, protein function, protein-protein interactions, or molecular transport with subcellular resolution. Fluorescence lifetime is a powerful tool that can provide this information. Traditionally, fluorescence lifetime techniques (such as FRET-FLIM) have been carried out on imaging platforms; however, the low throughput of microscopy severely limits the resulting efficiency of analysis.
Our newest product, the Danube II combines many of the benefits of FLIM analysis with the inherent high throughput of flow cytometry. The most advanced fluorescence lifetime flow cytometer on the market, it provides direct, time-domain analysis of fluorescence lifetime, with the ability to measure multi-exponential decay on a cell-by-cell basis at a throughput of up to 10,000 cells/second.
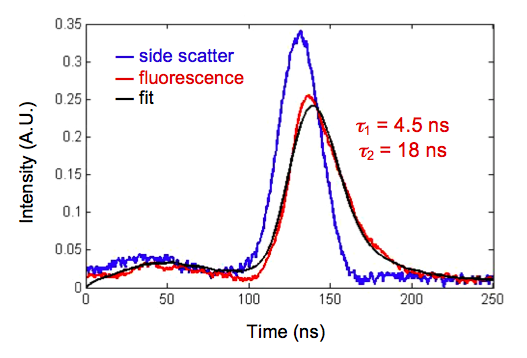
Danube I measurement of multiexponential fluorescence lifetime decay
of quenched ethidium bromide in CHO cells (Li et al., Proc. CYTO 2013).
The Danube II works by generating extremely short interactions between the interrogating laser light and the cells in the sample. Each cell is probed dozens of times, with each excitation event lasting as little as 5 ns. This capability, unique in flow cytometry, results in subnanosecond time resolution of fluorescence lifetime decay values, and the ability to measure lifetime changes in most of the fluorophores and fluorescent proteins in common use.
Working directly in the time domain, the Danube II is also capable of simultaneously resolving multiple lifetime components within the same cell. This allows the differential quantification of lifetime changes of a given compound in the subcellular environment.
The Danube II brings a new level of performance to cell analysis. By allowing the rapid measurement of subnanosecond lifetime changes across entire cell populations, it gives cell researchers a flexible and efficient new tool for the study of the subcellular environment.
 The Danube I. The footprint of the Danube II is 30-50% smaller,
The Danube I. The footprint of the Danube II is 30-50% smaller,
depending on the excitation and emission options.
The following are the Danube II‘s preliminary technical specifications*:
Excitation Optics
Standard laser:
* 488 nm, up to 150 mW
Optional lasers:
* 405 nm, up to 120 mW
* 532 nm, up to 300 mW
Emission Optics
Standard channels:
* FSC: 1 – 10°
* SSC: 90° ± 60°
* FL3: 530/30
* FL4: 580/30
Optional channels:
* FL1: 440/30
* FL2: 470/30
* FL5: 630/30
* FL6: 660/LP
Fluidics
Dual syringe drive injection
* Sheath: 10 mL
* Sample: 5, 10, 20, or 50 uL
* Injection speed: 0.1 – 60 uL/min
Built-in debubbling protocol
Signal Processing
Digital waveform sampling:
* 16-bit, 20 MS/s per channel
Full waveform storage:
* 2000 ms for single channel
* 250 ms for 8 channels
Optional signal analysis (offline):
* automatic multiexponential lifetime fit
* Flow Cytometry Standard (FCS) 3.0
Performance
Fluorescence lifetime:
* 5-ns interaction time
* 500-ps lifetime resolution
* multiexponential decay
Sensitivity (488-nm excitation, FL3 channel):
* FITC ≤ 500 MESF
* 6/8 Spherotech Rainbow bead peaks
* CV 6% (typ.)
Throughput:
* 10,000 events/s (typ.)
Installation Requirements
Dimensions:
* 20” x 25” x 12” (W x D x H)
Weight:
* 30 lbs. (1-laser, 4-channel system)
Environmental:
* 15° – 30°C, 60% RH
Power:
* North America: 120 VAC, 50/60 Hz, 5A
* Japan: 100 VAC, 50/60 Hz, 5A
* Rest of world: 230 VAC, 50/60 Hz, 3A
KRCDS.DanubeII.0v5
* For Research Use Only. Not for diagnostic use. Specifications subject to change without notice.